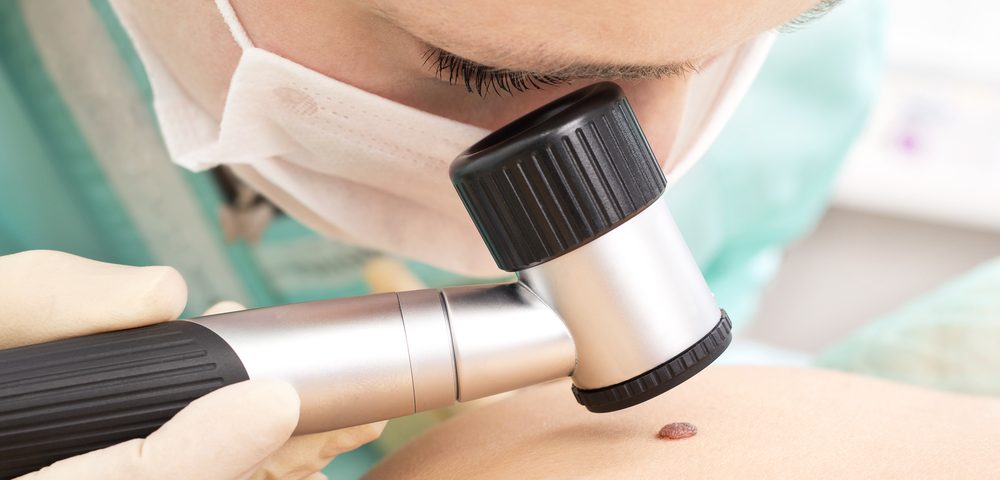
A group of experts have outlined recommendations for multiple melanoma screening to initiate the implementation of a national consensus on screening procedures. Currently there is no national screening guideline for melanoma, which leaves healthcare providers to decide on when and how often to screen for the disease.
The article, “Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy,” was published in the journal Melanoma Management. Over 50 dermatology and skin cancer experts participated in the perspective article.
The authors outlined three goals: to propose guidelines in line with the U.S. Preventive Services Task Force (USPSTF) screening guidelines for other disorders; to compare their proposed guidelines to other organizations; and to review the USPSTF 2016 Draft Recommendation Statement on skin cancer screening. Several points of criticism of this document were raised, hoping to start a debate that will lead to improved screening practices.
The expert panel, led by researchers at the Melanoma Research Program at Oregon Health & Science University’s Knight Cancer Institute, noted that despite research showing that total body skin examination is the safest and most economical melanoma screening method, how best to implement it is left to primary healthcare institutions.
“In many ways, it’s surprising that our field is currently without a national consensus for skin cancer screening guidelines for patients without symptoms,” Sancy Leachman, one of the two lead authors, said in a press release.
In addition to reviewing current research on screening practices, the research panel also looked at other countries with similar melanoma rates to gain insight from screening practices elsewhere (in particular, from Australia, New Zealand, and Germany).
“One of the goals of this paper was to propose data-driven, evidence-based guidelines for screenings that are consistent with the USPSTF guidelines for other cancers and diseases,” Leachman said. “The guidelines are of course just a starting point based on patient data we’ve reviewed to date, but we’ve identified a strong need to provide providers with initial recommendations outlining when to recommend screenings to their patients.”
Results from their data review led the group to recommend screening at least once a year using total body skin examination for adults ages 35 to 75 with at least one risk factor.
Risk factors included having a personal or family history of melanoma, a history of sunburns or indoor tanning use, or having certain physical attributes. Features that were considered to be at higher risk included blonde or red hair, fair or severely sun-damaged skin, many freckles, over 40 birthmarks, or more than two atypical birthmarks.
A key aspect of the article was the author’s critique and rationale of the USPSTF 2016 Sstatement on melanoma screening. Since its publication last year, the statement was not without criticism; by reviewing the document, the authors hoped to initiate a debate about its shortcomings.
The 2016 statement, for example, had omitted a delayed diagnosis as a link to morbidity in its risk estimates, and had extrapolated satisfaction results from cosmetic procedures to those procedures intended to diagnose cancer — one of the issues that the authors raised to challenge the document’s methodology.
The group also criticized the method used to gather the publications that formed the foundation of the statement’s reasoning.
“Given the current rates of melanoma diagnoses in the U.S., it is fascinating to see how the field continues to address screening inadequacies for a cancer with potentially such high mortality rates,” said Sebastian Dennis-Beron, commissioning editor for Melanoma Management.
“It has been a pleasure to work with Dr. Leachman and her colleagues to develop this timely and thought-provoking piece, as to really underline the need to develop evidence-based screening guidelines for melanoma patients, given how vital early detection is for survival.”