SciBase AB, a Swedish medical technology company headquartered in Stockholm, has recently announced the results of its US Reader Study. According to SciBase, the addition of Nevisense, which uses Electrical Impedance Spectroscopy (EIS) to categorize cellular structures and thereby detect malignancies, greatly improved the ability of dermatologists in accurately diagnosing melanoma. This reader study and its positive results fulfils the US Food and Drug Administration’s requirement of SciBase prior to filing a Pre-Market Approval (PMA) application.
“We are very pleased with the result of the study. This was the last requirement from the US FDA for our Pre-Market Approval (PMA) application for Nevisense on the US market. Now that the Reader study is successfully completed, we can finalize and submit our application”, says Simon Grant, CEO of SciBase.
The reader study enlisted the participation of 41 US dermatologists. They were asked to go online and review 141 randomly selected suspected melanoma lesions, initially only with patient information, and again with added Nevisense information. The study revealed the information from Nevisense better guided dermatologists to detect melanoma, successfully meeting the FDA’s preset primary endpoints.
“The study results are in addition to the results from the SciBase pivotal study published last year, but with a different approach and with a broad group of US dermatologists. This shows that Nevisense has the potential to provide additional valuable information to clinicians in the difficult task of accurately detecting malignant melanomas,” says Simon Grant, CEO of SciBase.
These results have given SciBase the green light to finalize its PMA application with the FDA, which is a requirement for the company to begin selling the medical device in the United States. SciBase is aiming to submit the PMA before the year ends.
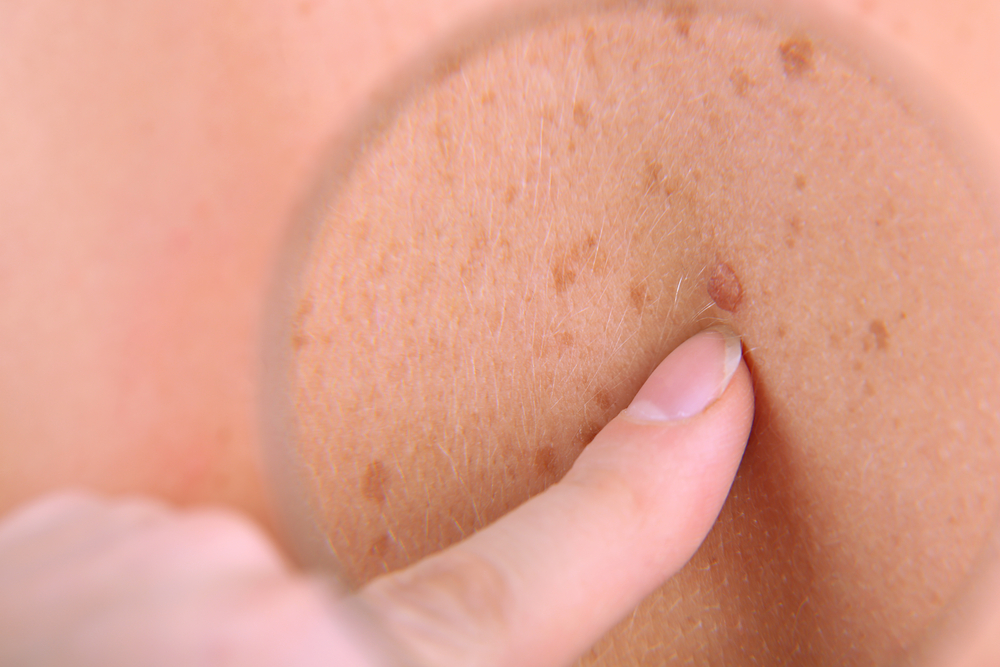
When it comes to predicting risk for skin cancer, many of us, including physicians, believe having more than 50 moles puts one at a much higher risk of developing melanoma. What most people fail to recognize is that there is still a need to be wary of skin cancer even with less than 50 moles. According to a recently presented study during the American Academy of Dermatology’s 2015 Summer Academy Meeting held in New York, individuals with fewer moles may be diagnosed with more aggressive melanoma than those with a higher number of moles. The study was led and presented by Caroline C. Kim, MD, FAAD, director, pigmented lesion clinic, and associate director of the cutaneous oncology program, Beth Israel Deaconess Medical Center Department of Dermatology, Harvard Medical School, Boston.