 Recent results from Provectus Biopharmaceuticals investigational drug PV-10 have been published in the Annals of Surgical Oncology.
Recent results from Provectus Biopharmaceuticals investigational drug PV-10 have been published in the Annals of Surgical Oncology.
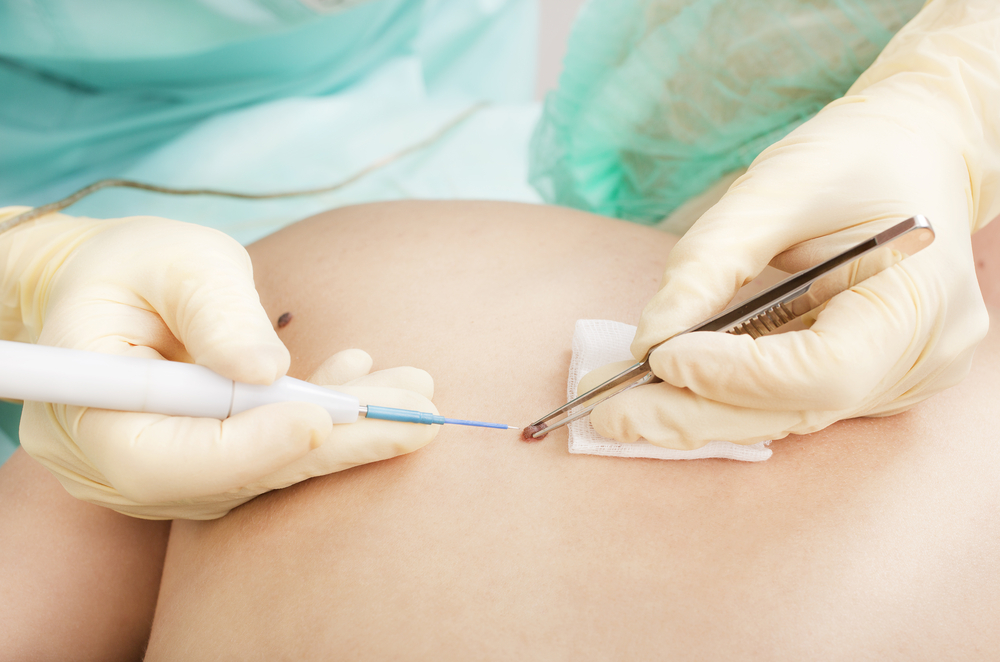
A team of research led by Eric A. Wachter, PhD, CTO of Provectus Biopharmaceuticals, Inc., evaluated the efficacy and safety of PV-10 (intralesional rose bengal) in 80 patients with refractory cutaneous or subcutaneous metastatic melanoma, in an international, multicenter, single-arm trial.
In the study “Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma”, the team treated 62 stage III and 18 stage IV melanoma patients disease refractory to a median of six prior interventions, with intralesional PV-10 four times over a 16-week period.
After following the patients for 52 weeks, the results showed the best overall response rate was 51 %, and the complete response rate was 26 %. Furthermore, the median time to response was 1.9 months, and median duration of response was 4.0 months, with 8 % of patients presenting no evidence of disease at the end of the 52 weeks.
John Glaspy, an oncology professor at UCLA, who was not involved in the study, told Forbes that it was not clear if these results were important. He continued saying that if the results were based on lesions that were not injected with the drug, they would be meaningful, however “If they are talking about the injected lesion, not so much. These SQ melanomas are an indolent disease, and it is not a big deal if you inject them and they regress. I don’t think you have any evidence that anybody is cured.”
As a reply to these previous comments, Dr. Wachter mentioned other cancer drugs that appeared to have extraordinary results at delaying disease progression, but did not cure the majority of patients. “I don’t want to get into an argument over the relative risks and benefits of such therapies vs. intralesional therapies, but will conclude by reiterating that patients clearly need more options: single agent options and more options for finding successful combinations that can truly change the course of this vicious disease,” he told Forbes.
Dr. Watcher further explained that upon injection of PV-10 in all of the patient’s skin malignancies, the response was usually faster and more dramatic, with an increased rate of complete response. “The patients may have benefited from more drug in some cases [of recurrence and partial response]. However, we need to prove that in phase III.”
Nonetheless, the team encountered some technical limitations in this study. For example, researchers could only treat 20 lesions per patient and only had three retreatment opportunities.
However, upon initiation of a Phase III trial, planned to begin by the end of 2014, these limitations will most likely disappear.